Issue 4: Vascular Specialisation and Barrier Function
This educational piece is designed for medical students and others interested in human physiology. Here, I introduce key concepts in the regulation and maintenance of the body’s fluid compartments
Issue 5: Vascular Specialisation and Barrier Function: Organ-Specific Capillary Architecture and Control of Plasma–Interstitial Exchange
The blood–brain barrier (BBB) is an emergent property of the specialised structure of cerebral microvessels, as established in previous work. These microvessels, composed of endothelial cells, pericytes, astrocytic endfeet, and supporting neural elements, collectively form the neurovascular unit (see Figure 1). Together, they give rise to the selective barrier properties characteristic of the BBB. However, the concept of a vascular barrier is not unique to the brain—it represents a universal principle of microvascular organisation throughout the body.
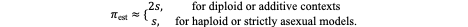
The circulatory system functions not only as a conduit for oxygen, nutrients, and signalling molecules but also as a dynamic interface between plasma and tissue microenvironments/compartments (see later). Each vascular bed is structurally and functionally adapted to the specific metabolic and physiological demands of its target tissue. This selective permeability is vital because the biochemical composition of plasma is not universally compatible with cellular homeostasis.
The brain provides the most striking example of this necessity. Blood plasma contains glutamate at concentrations several times higher than those tolerated within the brain’s extracellular space. It is interesting to note that glutamate is the primary excitatory neurotransmitter and one of the most abundant free amino acids in the brain, with an extracellular concentration of approximately 1–10 µM compared to 50–100 µM in plasma. Despite its ubiquity, even a modest increase in brain extracellular glutamate can be catastrophic. Say the brain’s interstitial fluid were to equilibrate directly with the plasma, such glutamate accumulation would be considered excessive, and it would trigger excitotoxicity due to overstimulation of glutamate receptors causing excessive calcium influx into neurons, activation of proteases and lipases, and ultimately neuronal death. So the role of barrier, particularly the blood brain barrier is of importance.
Amongst blood vessels, Capillaries Possess the Highest Cumulative Surface Area and Drive Most Exchange Through Functional Specialisation
As was implied in the introduction, the body's vascular network is generally a "space-filling" system i.e. it consists of branching parts which permeate tissues to ensure that no cell lies far from blood supply (see figure 2).
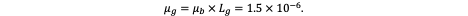
The structural composition of blood vessels, as shown in the figure above, varies according to their functional roles within the circulation. Among these vessels, capillaries provide the greatest cumulative surface area and serve as the principal sites of exchange between blood and tissue, thereby mediating the transfer of gases, nutrients, and metabolites. However, capillary architecture is not uniform; its anatomy differs across organs in accordance with local physiological demands.
In general, capillaries consist of a single layer of endothelial cells supported by a basement membrane and, variably, by pericytes and other cellular processes. But they exhibit considerable diversity in permeability properties, determined primarily by the presence or absence of fenestrations and by the tightness of intercellular junctions. To this end, we can classify capillaries into 3 broad categories (see figure 3).
Continuous capillaries, characterised by uninterrupted endothelium and tight junctions, are found in muscle, skin, lung, and the brain, and exhibit the most restrictive permeability. Fenestrated capillaries, such as those in the kidney glomeruli and endocrine glands, contain transcellular pores that enable high-volume exchange while maintaining selectivity against macromolecules. Finally, discontinuous (sinusoidal) capillaries, present in the liver, spleen, and bone marrow, feature wide intercellular gaps and discontinuous basement membranes, allowing the passage of cells and large proteins.
To further illustrate how vascular structure is shaped by organ-specific function, take the kidney, liver, and brain as examples. I have already used the glutamate example for the brain and will not reprise it here (see future newsletters). However the kidney’s primary role, rapid ultrafiltration of plasma, depends on a specialised glomerular capillary architecture.
Glomerular endothelial cells are fenestrated (pores ≈ 60–80 nm). This permits high-volume passage of water and small solutes while excluding cells and most large macromolecules. This permeable endothelium is overlaid by the glomerular basement membrane (a fused basal lamina rich in type IV collagen and heparan sulfate proteoglycans), which imposes both size- and charge-selectivity, and finally by podocyte foot processes whose slit diaphragms (nephrin-containing junctions) form the last physical sieve (see figure 4). The combination of large surface area, high hydraulic conductance (high Kf) and elevated glomerular hydrostatic pressure yields a large filtrate volume while preserving plasma proteins; disruption of any of these layers (loss of negative charge, slit-diaphragm defects, or GBM damage) produces proteinuria (protein in urine) - you'll see why this (proteinuria) can be problematic in a bit**.
In contrast, the liver demands extensive bidirectional exchange between sinusoidal blood and hepatocytes to sustain its roles in metabolism, synthesis, and detoxification (see figure 5). To accommodate this, hepatic sinusoids are discontinuous capillaries with wide (100–200 nm) intercellular gaps and a discontinuous or absent basement membrane, creating minimal resistance to macromolecular movement. The endothelial lining is fenestrated and lacks a diaphragm, enabling free passage of plasma proteins, lipoproteins, and metabolic substrates into the space of Disse, where they directly contact hepatocyte microvilli. Within this perisinusoidal space, Kupffer cells (resident macrophages) perform immune surveillance, while hepatic stellate cells regulate extracellular matrix composition and vitamin A storage. Together, this architecture creates a low-shear, high-permeability environment that supports the liver’s synthetic output and detoxification capacity, functions that would be impossible through the restrictive interfaces of continuous or fenestrated capillaries.
Movement across the capillary wall is either transcellular or paracellular
Consider the different ways molecules can cross the capillary wall. They either go through the transcellular route, i.e. they pass through endothelial cells (via diffusion, carrier-mediated transport, or vesicular mechanisms (transcytosis)) or the paracellular route where movement occurs between adjacent cells through the intercellular junctions, largely determined by the tight junction’s permeability (see figure).
The relative contribution of these pathways varies with tissue type. We broadly classify endothelial walls as “tight” or “leaky”, to reflect the degree to which paracellular diffusion is restricted. Leaky epithelia such as renal proximal tubules exhibit low transepithelial electrical resistance (around 6 Ω·cm²), whereas tight epithelia like the urinary bladder have resistances exceeding 70,000 Ω·cm². Importantly, the differences arise not from intrinsic variations in cell membrane resistance but from the properties of the tight junctions themselves. These junctions act as selective gates, with permeability varying to ions and solutes depending on tissue-specific protein composition (e.g., claudins, occludins). Regulation of this permeability allows tissues to maintain distinct luminal and abluminal environments, facilitating vectorial transport—the directional movement of solutes or fluids from one side of the cell layer to the other.
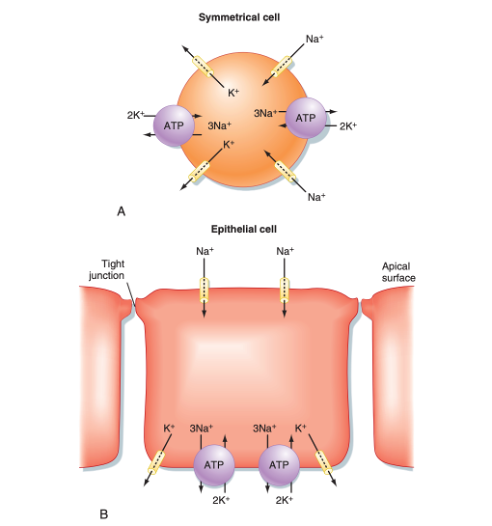
Endothelial cells are polarised with distinct apical and basolateral domains containing specialised transport systems
Tight junctions between endothelial cells restrict paracellular diffusion and thereby establish vectorial (directional) transport across the capillary wall (see figure). This structural organisation confers polarity to endothelial cells, creating apical (luminal) and basolateral (abluminal) domains that contain distinct complements of ion channels, pumps, and transporters. Such polarisation allows coordinated absorption, secretion, and selective transcytosis depending on tissue demands.
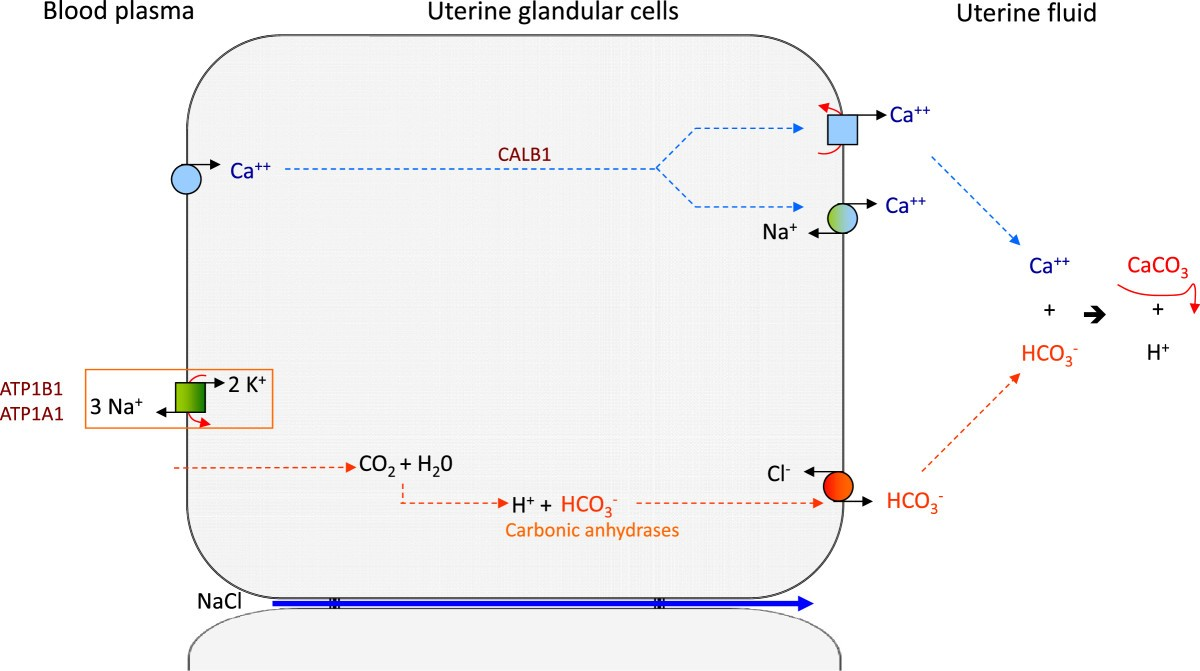
This asymmetry is fundamental because unlike erythrocytes, which merely traverse the circulation, endothelial cells define the partition between blood plasma and the interstitial space (figure 8). Through their polarised organisation, they maintain chemical gradients essential for homeostasis, regulating solute flux, ionic balance, and macromolecular exchange in a tightly controlled, tissue-specific manner.
The interstitial and plasma compartments
I have already mentioned that the capillaries separate the intravascular compartment from the interstitial compartments. At the cellular levels, the plasma membrane separates the intracellular from the extracellular environment (see figure 9).
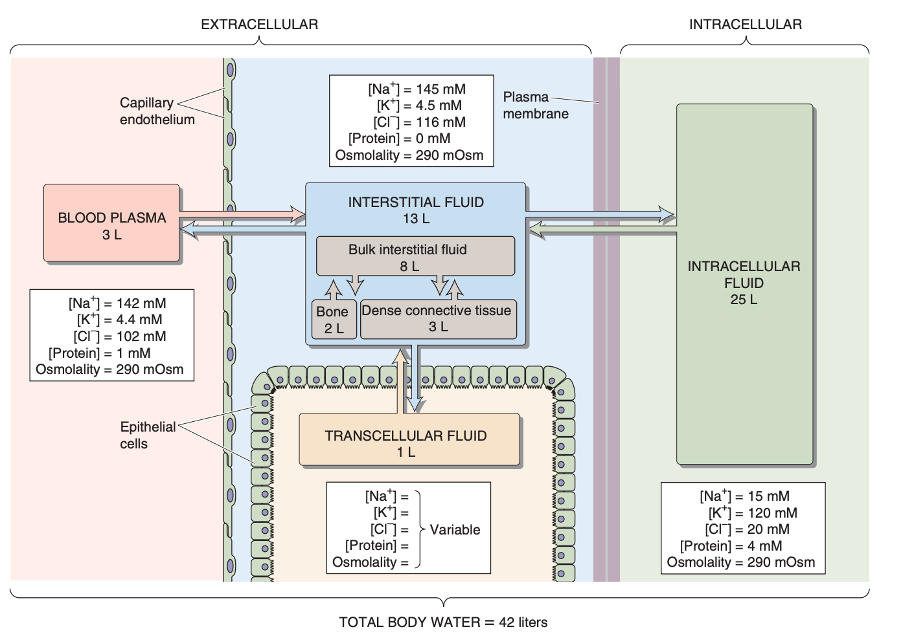
A fundamental property of fluid compartments is that they exist in osmotic equilibrium. This means that despite differences in solute composition, the total osmolality of plasma, interstitial, and intracellular fluids is nearly identical - and it has to be. Any perturbation in the osmolality of one compartment drives immediate water redistribution through aquaporins and other channels until equilibrium is restored. Thus, plasma osmolality serves as a reliable proxy for the osmotic state of all body fluids.
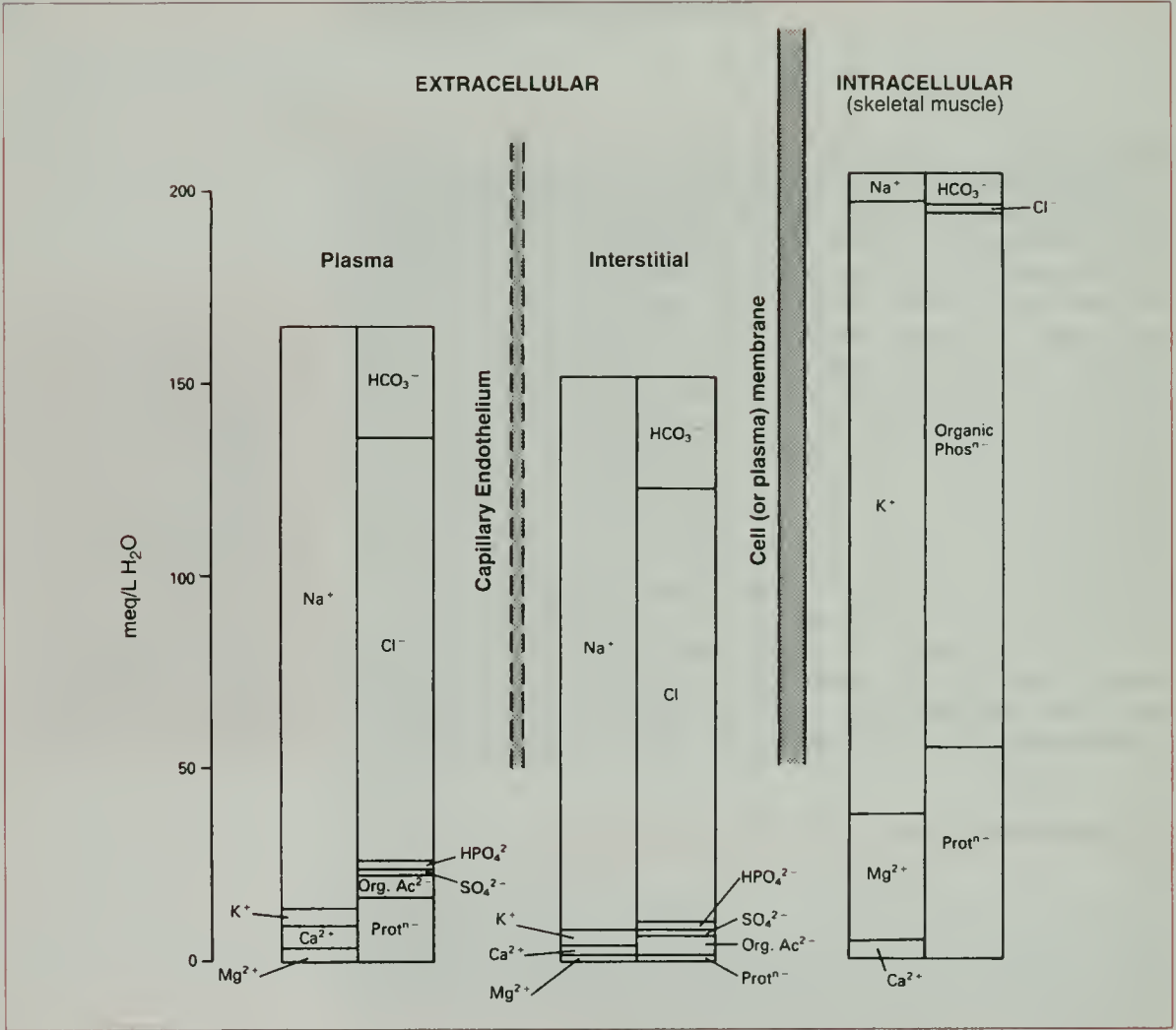
But since the capillary wall is selectively permeable, this osmotic equilibrium establishes what is called the Gibb-Donnan effect. Essentially this is a physicochemical principle which describes how impermeant charged molecules (such as plasma proteins) influence the distribution of permeant ions across a semipermeable membrane (like the capillary wall). Thus in the capillaries, the negatively charged plasma proteins, generate a slightly negative charge within the vascular lumen. This in turn draws additional cations (Na⁺, K⁺) into the plasma and excludes some anions (Cl⁻, HCO₃⁻), leading to a small but physiologically important difference in ionic composition between plasma and interstitial fluid (see following figure). The osmotic pressure arising from this protein retention is termed the oncotic pressure and plays a vital role in fluid balance across the capillary wall and underlies Starling’s forces.
The Starling equation, quantifies capillary fluid exchange as the net result of opposing hydrostatic and oncotic pressures across the capillary endothelium:
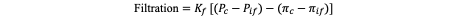

Disruption to this balance, presents clinically as edema i.e. the abnormal accumulation of interstitial fluid. Localised edema arises when one or more Starling forces are perturbed, for instance, in inflammation (increased Pc and Kf), lymphatic obstruction (impaired protein clearance, elevated πif), or venous congestion (increased Pc). Generalised edema, seen in cardiac, hepatic, or renal failure, reflects systemic sodium and water retention, leading to expansion of the entire extracellular fluid volume.
** Hence, this is why proteinuria is so problematic. When the kidney leaks protein into the urine, the impermeant solute fraction in plasma is lost, disrupting the local charge balance in blood vessels. The body compensates by redistributing ions - sodium in particular - thus altering plasma osmotic pressure, interstitial fluid composition, and overall fluid balance. This can lead to edema, shifts in blood volume (therefore, blood pressure), and downstream disturbances in cardiovascular and renal homeostasis.
In short, you need protein to trap sodium in the vessels, and sodium to trap water in the plasma - and all of this relies on the specialised structure of the renal capillaries.... isn't this just beautiful?