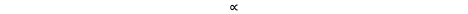Issue 5 — Tight junction Claudins
Claudins are the principal structural proteins of tight junctions. They dynamically regulate cell–cell adhesion, serving both as physical connectors and as selective channels that control paracellular ion flow. Altered claudin expression or function is implicated in various cancers.
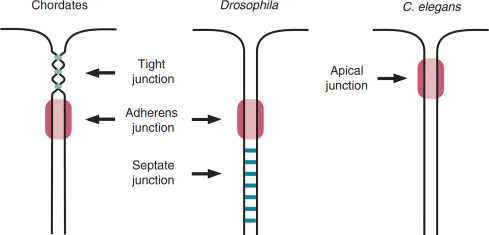
Cell–cell contacts underpin the higher-order organisation of multicellular life. As I outlined in previous newsletters, they are chiefly mediated by tight junctions and adherens junctions (there's others, but these will be considered elsewhere). Whilst these two types of junctions differ in morphology, composition and arrangement, I think their most striking distinction (to the point of definition) is in position. Tight junctions occupy the apical-most region of epithelial and endothelial interfaces whereas adherens junctions lie just beneath them. Most invertebrates lack canonical tight junctions and instead employ what are called septate junctions to fulfil similar barrier and adhesion roles. As you will see in the following figure these occupy a separate position to the tight junctions, this is why I said to the point of definition.
Tight junctions are multi-protein complexes composed of membrane-spanning and cytoplasmic adaptor proteins that assemble into a network of anastomosing, mesh-like strands encircling the apical region of epithelial cells. By sealing the intercellular cleft and defining the boundary between the apical and basolateral membrane domains, they maintain epithelial polarity and ensure the directional (aka vectorial) transport of substances essential for physiological homeostasis.
Box. 1 — Protein synthesis explained
Most of my discussion on this newsletter assumes a good level of understanding of protein synthesis. Thus this box should fill the gaps for those who may not have a background knowledge of this.
Proteins are the principal functional units of life. Examples include enzymes, receptors, channels, and structural scaffolds that underpin nearly every cellular process. Understanding their synthesis and folding provides the foundation for appreciating how proteins such as claudins build and regulate intercellular barriers.
From Gene to Protein
Protein synthesis begins with the genetic code encoded in DNA, which resides within the nucleus. The 'code' lies within the sequence of bases (A,C,G,T) that make up DNA. Because protein assembly occurs in the cytoplasm, the information stored in DNA must first be transcribed into a messenger RNA (mRNA) molecule — a process known as transcription.
DNA is composed of four nucleotides — adenine (A), thymine (T), cytosine (C), and guanine (G) — that pair via hydrogen bonding (A with T, G with C). The resulting double helix stores the template for every protein. During transcription, RNA polymerase reads one strand of DNA and produces a complementary RNA strand, substituting uracil (U) for thymine. Unlike DNA, RNA is typically single-stranded and structurally flexible, allowing it to exit the nucleus through nuclear pores.
Translation: Decoding the Message
Once in the cytoplasm, mRNA binds to ribosomes — large ribonucleoprotein complexes that serve as the molecular factories of translation. Ribosomes read the mRNA in triplets of nucleotides called codons, each specifying a single amino acid. Transfer RNAs (tRNAs), each carrying a specific amino acid, recognize codons via complementary anticodons and align them in sequence, forming a growing polypeptide chain. We define a peptide as a short chain of amino-acids linked together by peptide bonds, thus a polypeptide is many amino acids joined in this way. Motif refers to a recurring sequence or structural pattern in a protein which carries a particular functional or structural significance.
Translation can occur on free ribosomes (yielding cytosolic proteins) or on ribosomes bound to the endoplasmic reticulum (ER), producing membrane-associated or secretory proteins (claudin is a membrane associated protein and is produced in the ER). The sequence of amino acids generated — known as the primary structure — directly encodes the chemical potential for the final three-dimensional fold.
Protein Folding and Stabilization
Newly synthesized proteins rarely function as linear chains. They must fold into precise conformations determined by both local interactions (secondary structures) and global architecture (tertiary structure).
Two recurring structural motifs dominate early folding: α-helices and β-sheets. These are stabilized primarily by hydrogen bonds between backbone amide and carbonyl groups. The arrangement and interaction of these motifs define the overall tertiary structure, which dictates the protein’s function.
Folding is driven by a combination of non-covalent forces which collectively stabilise the protein’s architecture:
· Hydrophobic interactions: Non-polar amino acid side chains (e.g., leucine, valine, phenylalanine) cluster toward the protein’s core to avoid contact with water. This “hydrophobic effect” is the major thermodynamic driving force for folding, contributing roughly 60–80 kJ/mol in stabilization energy.
· Van der Waals forces: Weak, transient attractions between atoms (~1 kJ/mol each) become collectively strong in the densely packed interior of proteins, complementing hydrophobic packing.
· Electrostatic interactions and salt bridges: Charged side chains (e.g., lysine, glutamate) form longer-range ionic attractions that constrain backbone motion and reinforce tertiary structure. Their contribution depends strongly on local dielectric environment and neighboring charges.
· Hydrogen bonds: Directional and specific, they provide 8–29 kJ/mol of stabilization and are crucial for structural specificity. Nearly 90% of backbone CO and NH groups participate in such bonding, ensuring internal cohesion.
· Disulfide bonds (S–S): Covalent links formed between cysteine residues under oxidizing conditions, disulfide bonds confer exceptional stability, especially in secreted and extracellular proteins such as claudins.
· Metal cofactors and prosthetic groups: Some proteins, such as zinc-finger domains or heme-containing enzymes, require bound metal ions or organic cofactors to stabilize folding and confer catalytic or structural function.
The interplay between these interactions allows proteins to achieve a thermodynamically stable “native” state. Folding is not purely spontaneous; cellular chaperones and the ER quality control system assist in proper folding and prevent aggregation, ensuring only correctly folded proteins proceed to function.
From Folding to Function
Ultimately, protein structure dictates function. The linear amino acid sequence (primary structure) determines the pattern of local folds (secondary structure), which collectively form the tertiary and, in some cases, quaternary architecture of multi-subunit complexes. This hierarchical organization underlies how proteins such as claudins assemble into highly ordered tight junction strands that regulate paracellular transport and maintain epithelial polarity.
The three main components of tight junctions are the claudin family of proteins forming the structural backbone of intercellular sealing strands; the TAMP family (including occludin, tricellulin, and other MARVEL-domain proteins) and the cytoplasmic scaffold proteins ZO-1, ZO-2, and ZO-3, which anchor these transmembrane elements to the actin cytoskeleton and integrate signalling cascades involving kinases and GTPases (see figure 1). Although all of these will be explored, I will mainly focus my attention on the claudins and discuss all other components in relation to them.
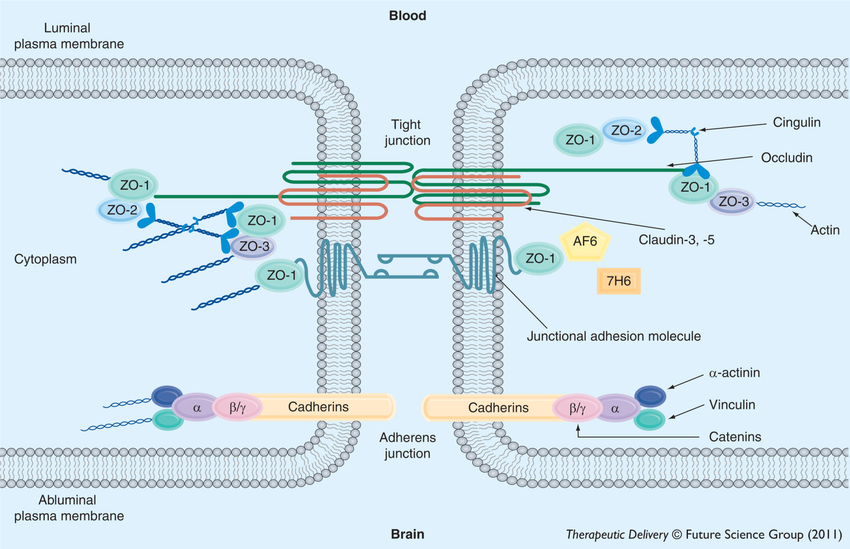
To justify my focus on claudins, consider that tight junctions are a characteristic feature of epithelial cells not typically expressed in cells of mesenchymal origin like fibroblasts. However, ectopic expression of claudins in fibroblasts is sufficient to drive the de-novo assembly of continuous tight junction like strands [1]. Thus it seems that claudins can nucleate junctional strand formation and define membrane microdomains necessary for tight junction assembly.
Different claudins are variably expressed in different tissues and they display remarkable functional diversity. Claudin-5 is enriched in cerebral endothelial cells (issue 3) and confers the blood brain barrier with its extraordinary restriction of ionic flow (discussed in issue 3). So too does claudin-1 expressed in the skin which maintains the skin barrier. Both of these claudins generate high-resistance barriers which are impermeable to both cations and anions. In contrast, claudin-2 expressed in the kidney and intestinal epithelia form cation-selective channels whilst claudin-4 favours anion permeability[2].
These apparently unique biophysical properties can be attributed to slight variations in the claudin protein structure as discussed later.
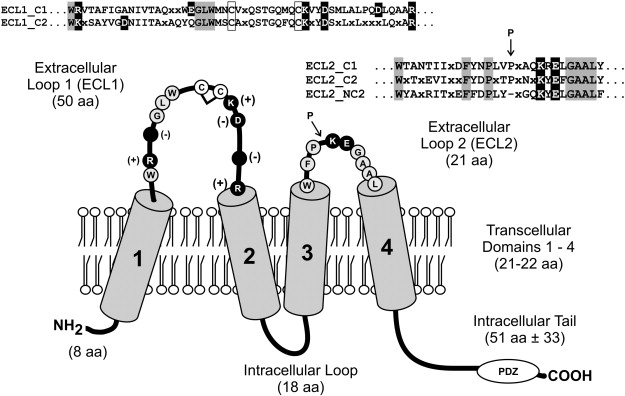
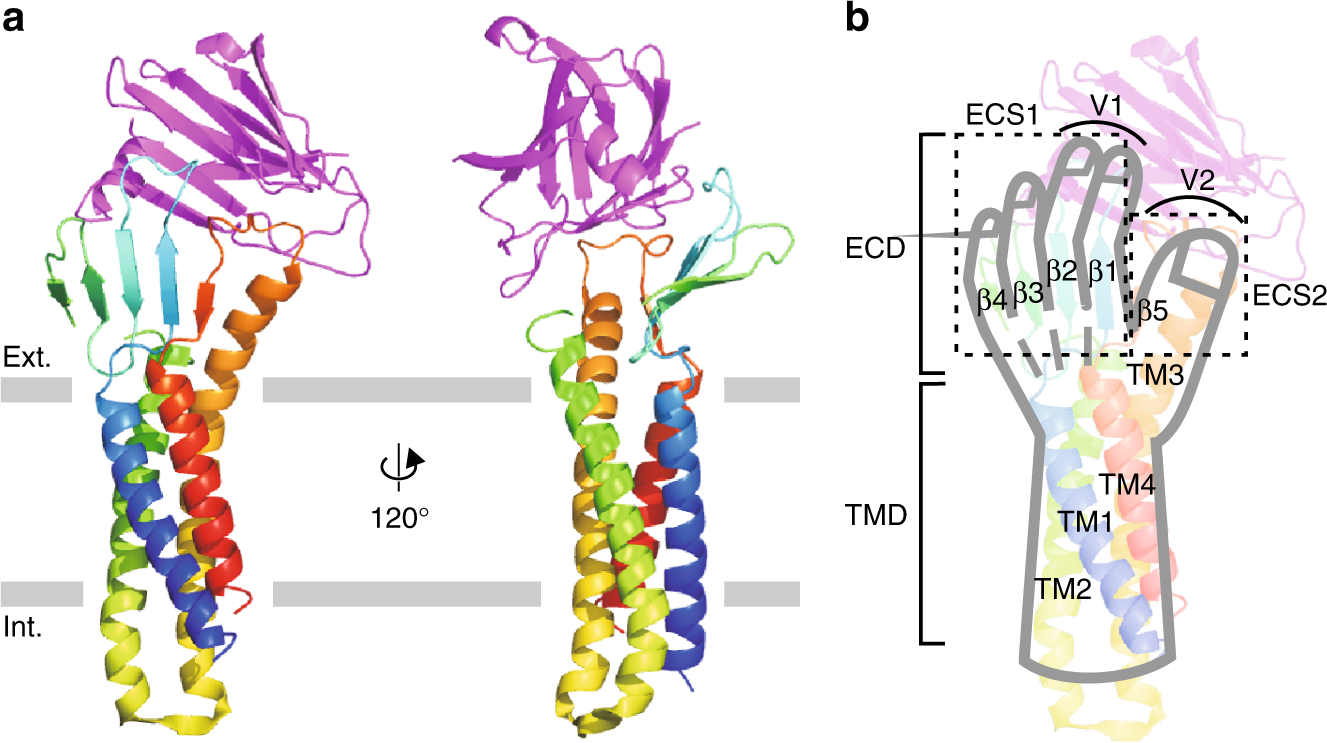
Generally, claudins span the membrane four times with two extracellular loops (ECLs, call these ECL1 and ECL2) and a shorter intracellular loop (see figure 3). Their N terminus and C terminus sit intracellularly. The C terminal is PDZ-binding and thus engages adaptor proteins like ZO-1, 2 and 3 as well as MUPP1 which tether the tight junctions to the cell’s cytoskeleton and also allow signal transduction. Although there still exists some disputations with regards to the tertiary and quaternary structures of claudins, some points about it structure can be made[3].
The extracellular domain
The two extracellular loops of claudins, ECL1 and ECL2 define the architecture, ion selectivity and the adhesive interface of tight junction strands. ECL1 is the longer of the two. It is approximately 50 amino acids long and contains signature sequences which mediate interactions between claudin family members, thus promoting tightening of the paracellular cleft, ion pore formation and sealing functions which decrease paracellular ion permeability.
In ECL1, the claudin family share a highly conserved signature sequence GLW [X]2-C-[X]8–10-C (see Box. 2).
Box. 2 — GLW [X]2-C-[X]8–10-C
GLW [X]2-C-[X]8–10-C is code for the consensus sequence found within the first extracellular loop of claudins. It is a mnemonic that describes the different amino acids in this sequence;
- G = Glycine
- L= Leucine
- W= Tryptophan
- [X]2 = 2 variable amino acids which vary amongst different claudins
- C = Cysteine
- [X]8–10 = Variable sequence of amino acids (~8-10 of them)
- C = Cysteine
These structures are evident in figure 3. GLW and the two cysteines are invariant in most claudins hence the terms "conserved", "signature"
The GLW tripeptide is a conserved signature motif of the claudin family, positioned at the N-terminal region of ECL1, immediately upstream of a pair of invariant cysteines. These cysteines form intramolecular disulfide bonds that stabilize the loop conformation, a structural feature essential for maintaining the architecture of the paracellular cleft (see Fig. 3). Notably, the GLW motif is conserved across nearly all vertebrate claudins (claudin-1 through claudin-23), highlighting its fundamental role in loop integrity rather than in isoform-specific functional specialization[4] [5].
Structurally, the small glyceine offers local conformational adaptability, leucine contributes hydrophobic packing that stabilizes the β-turn and tryptophan with its bulky aromatic ring provides a hydrophobic anchor which facilitates the correct folding of ECL1 against the transmembrane helices. This combination creates spatial alignment that is necessary for trans-interactions between opposing cells[6].
Immediately downstream of the GLW motif lies the cysteine residues which form an intramolecular disulfide bridge that stabilises the tertiary fold of ECL1. The disulfide bond, it is said, helps to maintain the correct orientation of the extracellular loop relative to the transmembrane helices thereby ensuring proper alignment of paracellular pores and promoting the tight, strand-like organization characteristic of functional junctions[6].
Much less is known about the structure of ECL2. However, it is thought to serve as the principal adhesive interface mediating trans-interactions between claudins on opposing cell membranes (see Box. ). When expressed recombinantly as a fusion with maltose-binding protein, the ECL2 of claudin-5 spontaneously dimerises—an intrinsic property that reflects its adhesive potential. In an elegant and systematic mutagenesis study, Piontek et al. substituted each residue within claudin-5 ECL2 and expressed the variants in HEK cells. Mutations at specific sites disrupted claudin-5 enrichment at intercellular contacts while leaving cis associations (measured by FRET) intact, indicating that these residues are critical for trans-binding across the paracellular space.
Box. 3 — TEER and tight junctions
Suppose a simple sheet of epithelial cells. This sheet separates two compartments of the body (for example, blood and lumen), and substances can cross it via two main routes: through the cells (the transcellular pathway) or between the cells (the paracellular pathway)
From an electrical standpoint, this system behaves like a circuit composed of resistors. The total transepithelial resistance (Rt), a measure of how easily ions can pass across this epithelial sheet, can be thought of as a sum of the two components;
- The transcellular resistance (Re), which includes the resistances of the apical and basolateral membranes (Rₐₘ and Rbₘ, respectively).
- The paracellular resistance (Rp), which includes the resistance of the tight junction itself (Rⱼ) and that of the intercellular cleft (Rᵢ).
Thus, the total resistance of the epithelium depends on whether current prefers to flow through cells or between them. In "leaky' epithelia, like the kidney, the paracellular pathway dominates. In tight epithelia, say the blood brain barrier, most current passes transcellularly.
Now say we have some freeze-fracture electron microscopy images which show tight junction strands. If the strands behaved as simple resistors in series, resistance would scale linearly i.e. if each strand observed acted as a fixed, impermeable wall, resistance would scale directly to strand count. But I have already mentioned above that it experimentally does not. Rather junctional resistance increases exponentially with the number of strands.
Phillipe Claude reasoned that a possible physical basis for this non-linear relationship "might be that the strands contain labile porelike structures that can be either open or closed to the movement of small ions[15]."Thus, if each claudin strand contains transient aqueous pores that flicker between open and closed states, the fraction of time a pore remains open is described by a probability p. The total junctional resistance, therefore, depends not only on how many strands (n) exist but also on how frequently their pores are open:
Rⱼ ∝ p-n
When p = 1 or n = 0, the junction behaves like an open slit with minimal resistance (~3.4 × 10³ Ω·cm). As p decreases (i.e., pores close more often), Rⱼ rises exponentially. This model fits experimental data remarkably well (correlation coefficient r ≈ 0.93), suggesting an average open probability of p ≈ 0.43—meaning roughly 43% of pores are open at any given time. Notably, even small changes in p dramatically alter total resistance: in an eight-stranded junction, reducing p from 0.4 to 0.3 can increase Rⱼ almost tenfold.
We know today that tight junctions are not static barriers but dynamic, ion-selective interfaces. Molecular and electrophysiological studies have demonstrated that expressing specific claudins in otherwise non-epithelial or claudin-deficient cells reproduces charge-selective permeability profiles characteristic of native epithelia [5, 16]. Claudins form paracellular ion channels whose selectivity and conductance depends on their isoform composition and assembly dynamics.
Claudin extracellular loops and channel function
I have probably mentioned this in previous newsletters but lets properly introduce the concept of transendothelial electrical resistance (TEER). TEER serves as a quantitative proxy for barrier integrity. It reflects the ability of tight junctions to restrict paracellular ion flux across epithelial or endothelial monolayers. We know from electrophysiology studies that the formation of tight junctions coincides with a non-linear increase in TEER (the relationship is logarithmic).
One way to perceive of the pore-forming function of claudins is as an emergent property of their extracellular architecture (primarily encoded within the ECL1). When two opposing strands meet from adjacent cells, their ECL1 β-sheets interdigitate to form a β-barrel-like structure that defines the paracellular pore. Using the crystal structure of claudin-15 as an example, one can note that ECL1 folds into a four-stranded antiparallel β-sheet, capped by a short β-strand contributed by ECL2 [7]. Together, these five β-strands form the extracellular wall of the paracellular channel. In this arrangement, the side chains of certain ECL1 residues—particularly those adjacent to the second conserved cysteine of the GLW–C–C motif—face the aqueous lumen of the pore, thereby determining ion selectivity. Thus, in claudin-2 the negatively charged Asp65 (D65) adjacent to the second conserved cysteine defines the cation selectivity of this claudin [8]. In claudin-15 the homologous Glu64 (E64) creates a ring of negative charge that electrostatically favours Na and other cations.
The intracellular domain
As shown in figure 3, the intracellular loop between the second and third transmembrane components is short. It acts as a rigid connector that positions the third and fourth transmembrane proteins and the extracellular loops. Thus, mutations here perturb strand assembly indirectly by changing TM helix register [9]. The N-terminus is short too, poorly conserved and cytosolic. It contributes little to scaffolding but can influence oligomeric packing and ER quality control in some isoforms[10].
The C-terminal (COOH) tail is the regulatory epicentre: typically 20–60 residues, intrinsically disordered, and harbouring (i) a juxtamembrane trafficking motif (~first 15–20 aa), (ii) membrane-proximal cysteine pairs subject to S-palmitoylation, and (iii) a terminal PDZ-binding motif (usually –YV). Discrete sequence differences in this tail explain major isoform-specific behaviours (trafficking, half-life, regulatory phosphorylation)[11].
Truncation or deletion of the proximal C-terminal residues causes ER retention and proteasomal degradation for multiple claudins (claudin-1, -5, -6, -16), demonstrating that the tail contains determinants required for productive folding/ER exit rather than merely for junctional anchoring. These signals operate independent of the distal PDZ motif, implying an early, tail-proximal chaperone or COPII-engagement function[12].
The terminal PDZ-binding dipeptide engages PDZ domains in ZO-1, ZO-2, ZO-3 and other multi-PDZ scaffolds (MUPP1), creating a physical bridge to F-actin and to signalling modules (GTPases, kinases). PDZ docking concentrates claudins in the apical junctional complex and enhances strand stability and lifespan; PDZ disruption more commonly accelerates turnover than prevents initial targeting. Visual, biochemical and mutational analyses (including ZO-1 deletion/PDZ competition) demonstrate this scaffolding axis as essential for dynamic coupling of strands to the cytoskeleton[13].
Conserved cysteines proximal to TM4 are S-palmitoylated (e.g., claudin-14; broader family evidence available). Palmitoylation increases membrane affinity, promotes partitioning into junctional microdomains and improves TJ localization efficiency — loss of palmitoylation reduces junctional enrichment and TEER even if strand-assembly competence remains[14]. Thus, palmitoylation is a reversible trafficking/stabilization code.
References
1. Furuse, M., et al., Conversion of Zonulae Occludentes from Tight to Leaky Strand Type by Introducing Claudin-2 into Madin-Darby Canine Kidney I Cells. The Journal of Cell Biology, 2001. 153(2): p. 263-272.
2. Amasheh, S., et al., Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. Journal of Cell Science, 2002. 115(24): p. 4969-4976.
3. Meoli, L. and D. Günzel, The role of claudins in homeostasis. Nature Reviews Nephrology, 2023. 19(9): p. 587-603.
4. Van Itallie, C.M. and J.M. Anderson, CLAUDINS AND EPITHELIAL PARACELLULAR TRANSPORT. Annual Review of Physiology, 2006. 68(Volume 68, 2006): p. 403-429.
5. Furuse, M., et al., Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. The Journal of Cell Biology, 1998. 141(7): p. 1539-1550.
6. Gonschior, H., et al., Nanoscale segregation of channel and barrier claudins enables paracellular ion flux. Nature Communications, 2022. 13(1).
7. Suzuki, H., et al., Crystal Structure of a Claudin Provides Insight into the Architecture of Tight Junctions. Science, 2014. 344(6181): p. 304-307.
8. Li, J., et al., Comprehensive Cysteine-scanning Mutagenesis Reveals Claudin-2 Pore-lining Residues with Different Intrapore Locations. Journal of Biological Chemistry, 2014. 289(10): p. 6475-6484.
9. Kausalya, P.J., Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. Journal of Clinical Investigation, 2006. 116(4): p. 878-891.
10. Koval, M., Differential pathways of claudin oligomerization and integration into tight junctions. Tissue Barriers, 2013. 1(3): p. e24518.
11. Rüffer, C. and V. Gerke, The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. European Journal of Cell Biology, 2004. 83(4): p. 135-144.
12. Koval, M., Claudins—Key Pieces in the Tight Junction Puzzle. Cell Communication & Adhesion, 2006. 13(3): p. 127-138.
13. Van Itallie, C.M., A.J. Tietgens, and J.M. Anderson, Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Molecular Biology of the Cell, 2017. 28(4): p. 524-534.
14. Van Itallie, C.M., et al., Palmitoylation of claudins is required for efficient tight-junction localization. Journal of Cell Science, 2005. 118(7): p. 1427-1436.
15. Claude, P., Morphological factors influencing transepithelial permeability: A model for the resistance of theZonula Occludens. The Journal of Membrane Biology, 1978. 39(2-3): p. 219-232.
16. González-Mariscal, L., et al., Tight junction proteins. Progress in Biophysics and Molecular Biology, 2003. 81(1): p. 1-44.