Introduction to Neuroscience — Issue 1
Hello all, welcome to this new weekly newsletter where I will endeavour to describe and make more accessible neuroscientific and oncological knowledge and findings.
The goal is to create discussion related to these topics and perhaps inspire people with regards to the importance of these topics in our world.
One of the most effective ways to grasp a field and the thinkers who shaped it, is to examine the tools at their disposal. Therefore, in this first letter, I seek to introduce some of the key neuroscientific tools currently being employed to help advance our understanding of the brain. This is purely going to be a consideration of only biochemical assays.
What is neuroscience?
Simply put, neuroscience is the study of brain function. It entails insights into the ‘hardware and software’ of the brain. But even more than that neuroscience is the science that informs our understanding of behaviours. It helps us unchain the links in the patterns that result in different human reactions and interactions. Thus, at both the economic level and the individual level, neuroscience is of the utmost relevance. In this first newsletter I will introduce you to the current methods used in the study of neuroscience. It is important to note that this is only an introduction to the field and therefore only superficial descriptions of these methodologies will be provided. I assume basic understanding of the structure and components of neurons.
Methods in neuroscience
Neuroscience is a field that has traditionally been grounded in anatomy. Galen’s principle that structure follows function has for example endured to modern microscopic methods1 2. However, beyond this reliance on anatomy, modern neuroscience also encompasses biochemical approaches targeting proteins, genomic analyses of expression and regulation, and electrophysiological methods that resolve the dynamics of neuronal activity.
Here are some of the methodologies:
Histology and Tract-Tracing Methodologies in Neuroscience
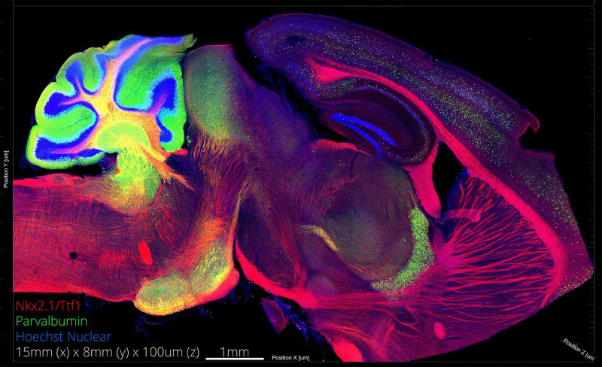
We have histology and microscopy to thank for many of the most striking, almost artistic images of the brain (see figure 1). When it comes to the studies of brain structure and function, histology is both fundamental and simple to grasp. It involves harvesting the brain from an organism, fixation, pre-embedding preparation, and embedding in paraffin, gelatin (or agarose), or preparing frozen sections via cryostat. The embedded tissue is then sectioned (‘microtomed’) and stained in preparation for imaging by light or electron microscopy.
Today, immunofluorescent or immunohistochemical stains are most commonly employed. Both are based on the immunological principle of antigen-antibody specificity. They involve the binding of a specific antibody to a relevant antigen, followed by visualization through either a fluorescent dye-conjugated antibody or a reaction product revealed by histochemical staining. While a detailed discussion of these methods lies beyond the scope of this paper, familiarity is fundamental to appreciating the breadth of neuroscience.
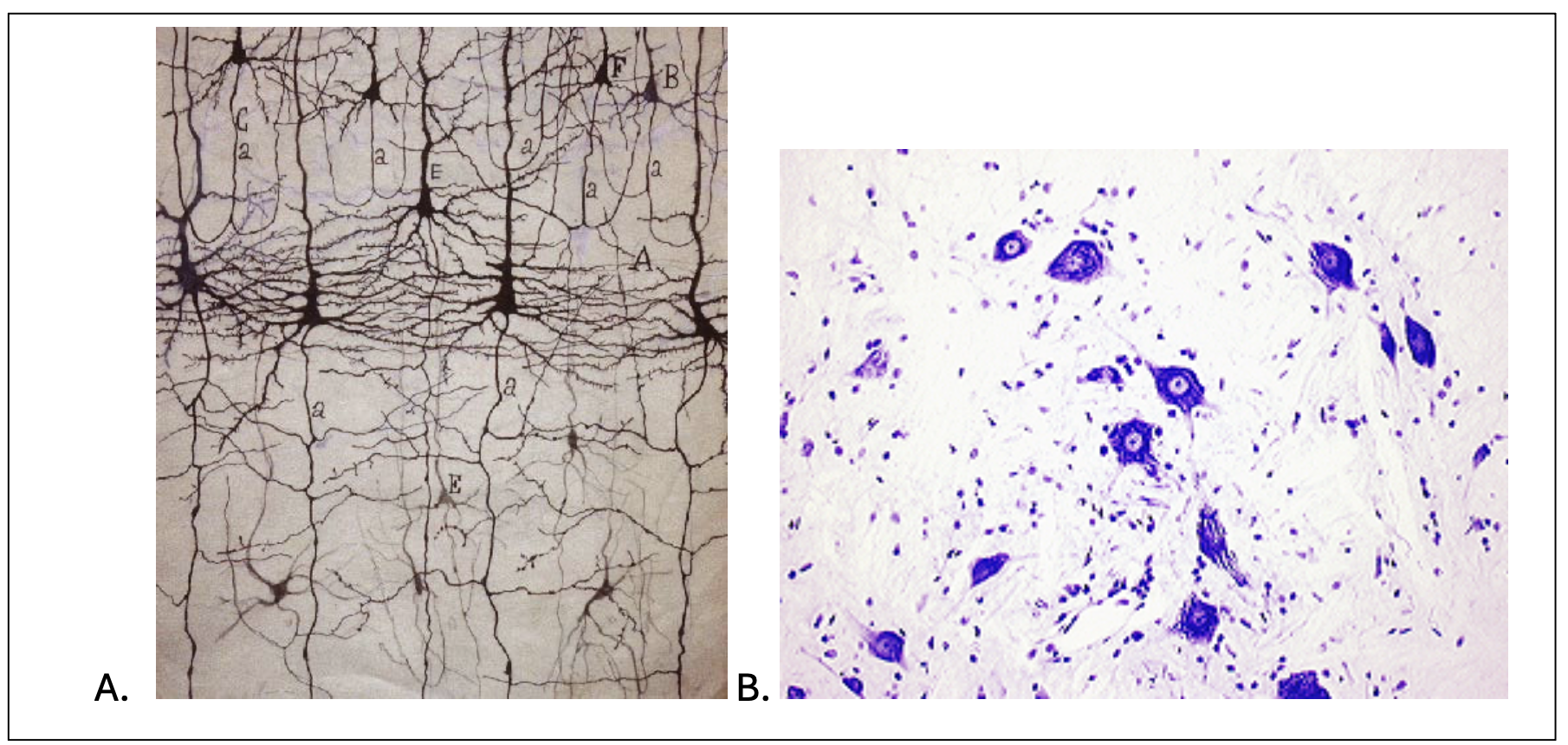
Two classical stains, however, merit mention for their historic impact: the Golgi and Nissl stains. The Golgi stain is able to selectively label the soma, dendrites and axons of a small subset of neurons in a large neuronal population. It’s mechanism of action is still not fully understood. However, it allows scientists to view, with exquisite detail, the dendritic arbour and axons of individual neurons with minimal overlap and or artefactual interference. Indeed, the ability to resolve individual neurons revolutionized neuroanatomy in the 19th century and enabled morphological classification of the neurons (figure 2a). Nissl staining, by contrast, targets the negatively charged molecules within Nissl bodies. Nissl bodies can be seen as granular structures composed of rough endoplasmic reticulum. Thus, Nissl stains allow the soma of neurons to be readily distinguished from glia (see figure 2b). Although both Golgi and Nissl stains are now largely of historical interest, superseded by modern immunofluorescent and immunohistochemical approaches, they laid critical conceptual and methodological foundations for the field of neuroscience.
A major component of modern histological analysis is image processing. However, this is a topic that is too extensive to explore here.
Neuronal tract tracing builds on basic histological staining to map connections between neuronal populations. The two main types are anterograde tracing which follows axons to their synaptic targets and retrograde tracing which reveals sources of input. Traditionally both of these techniques were performed with chemical tracers, but now viral vectors are increasingly being used3. This, in parallel with, high resolution imaging and 3D digital reconstructions of labelled neurons integrated with computational modelling forms the basis of the field of connectomics.
In Vitro Neural Preparations: Cell Isolation and Quantitative Analysis via Flow Cytometry
A large portion of neuroscience research is performed in vitro. For this, one must first isolate cells from model animals. Various in vitro preparations exist, though their detailed description is beyond the scope of this paper. Commonly isolated cells from the brain and CNS include hippocampal neurons, microglia, astrocytes, neural stem/progenitor cells, among others. Remarkably, even mitochondria can be isolated from brain tissue, which is particularly relevant in neurodegenerative diseases such as Parkinson’s, Huntington’s, Alzheimer’s, and acute myeloid leukemia.
Flow cytometry is widely used to sort peripheral immune cells from other neural cell populations. The principle is straightforward: a laser passes through a salt-buffered suspension of cells, measuring light scatter. Forward scatter reflects cell size, while side scatter reflects granularity with both types of scatters being independent of fluorescence. Researchers can also fluorescently tag specific proteins to quantify surface or intracellular markers at single-cell resolution. This allows identification and isolation of neuronal subtypes, glial populations, or stem-cell-derived lineages for downstream analyses such as transcriptomics or functional assays. We call this process, fluorescence-activated cell sorting and it involves fundamentally speaking, charging fluorescently tagged cells as they pass through the laser. Charged deflection plates direct the cells into collection tubes, isolating highly specific populations. The results are then typically visualized as dot plots comparing two variables, with each dot representing a single cell and the distribution revealing subpopulations distinguished by marker expression.
Gene expression analyses
Gene and protein expression analyses are widely employed in neuroscience. Examples of such methods include western blotting which detects specific proteins by transferring electrophoretically separated proteins onto a membrane and probing them with antibodies, enabling identification, separation, and localization. PCR-based methods similarly allow precise analysis of gene expression. As these are standard biochemical techniques with no particular uniqueness to neuroscience, they are assumed knowledge and will not be discussed further.
Nanotechnology in neuroscience
Nanotechnologies involve engineered materials or devices with at least one dimension on the nanometre scale (1–∼100 nm), giving rise to functional properties not present in their constituent elements. Their defining feature is that their nanoscale design confers unique optical, electrical or mechanical properties which thereby enable interactions that may not be achievable by bulk materials. In neuroscience, nanoengineered materials can promote neuronal adhesion and growth or enhance technologies that interface with neurons in vivo, such as electrode coatings4. Key challenges include achieving specificity, inducing desired physiological effects, and minimizing side effects, compounded in vivo by the complexity and anatomical constraints of the CNS. Despite these hurdles, nanotechnologies hold significant potential for advancing our understanding of nervous system function, disease mechanisms, and molecular-level interventions.
Electrophysiology
Electrophysiology is a core field in neuroscience that studies the electrical activity of neurons and the molecular and cellular processes underlying their signalling. It measures voltage changes and currents in neurons and circuits, including membrane potentials, action potentials, synaptic potentials, and ion channel dynamics, providing direct insight into neural communication. One common electrophysiology method is extracellular recording, where electrodes placed outside cells detect action potentials and local field potentials from neuronal populations. Another is patch-clamp recording, which uses a micropipette to isolate ionic currents with high sensitivity, allowing measurement of single-channel activity, whole-cell conductance, and subthreshold events with exceptional resolution
Finally, it is worth noting the role of behavioural studies in neuroscience, which are among the most analytically challenging. Many difficulties arise from determining which parameters are truly relevant. Behavioural neuroscience encompasses motor and sensory studies, as well as aspects of psychology and psychiatry. While these areas are not discussed here, they remain a crucial component of the broader field.
References
1. Kühn, K.G. Claudii Galeni Opera Omnia, (Cambridge University Press, Cambridge, 2011).
2. Crawford, L.K. & Caterina, M.J. Functional Anatomy of the Sensory Nervous System: Updates From the Neuroscience Bench. Toxicologic Pathology 48, 174-189 (2020).
3. Xu, X., et al. Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers. Neuron 107, 1029-1047 (2020).
4. Shaikh Mohammed, J., DeCoster, M.A. & McShane, M.J. Micropatterning of Nanoengineered Surfaces to Study Neuronal Cell Attachment in Vitro. Biomacromolecules 5, 1745-1755 (2004).